Pharmaceutical Production Methods
Ciprofloxacin Synthesis
Streamlined synthesis of antibiotic ciprofloxacin
Ciprofloxacin is the most widely prescribed fluoroquinolone class of antibiotics and is used to treat gastrointestinal infections and urinary and respiratory tract infections. Since its discovery few changes have been made to its general synthetic scheme. Synthesis of active pharmaceutical ingredients (API) account for the highest proportion of total drug product cost, limiting access to global health care management. Many of API process develop from medicinal chemistry routes designed for fast commercialization, leaving many of these formulations with the high potential for applying new synthetic approaches.
The technology
A method has been developed that improves on current processes to synthesize ciprofloxacin by starting with a more affordable starting material (Figure 1. Compound 3). This method significantly improves on the current ciprofloxacin synthesis process by reducing the number of steps to three high yielding reactions. An additional reduction in cost comes from the development of a continuous flow process with fewer reactors and unit operations. This “synthon” preparation allows for earlier insertion of the cyclopropylamine moiety (2). A chemoselective C-acylation of the enamine can be achieved at a high yield to produce a key intermediary (5). Specifically, the nucleuophilic displacement of the vinyl ether by cyclopropylamine to produce the enamine (3). This synthon is C-acetylated to 2,4-dichloro,5-fluoro benzoyl chloride in the presence of LiHMDS followed by its intramolecular nucleophilic substitution in the presence of DBU to produce (6). Piperazine coupling and subsequent hydrolysis of the ester produce the desired ciprofloxacin product (1).
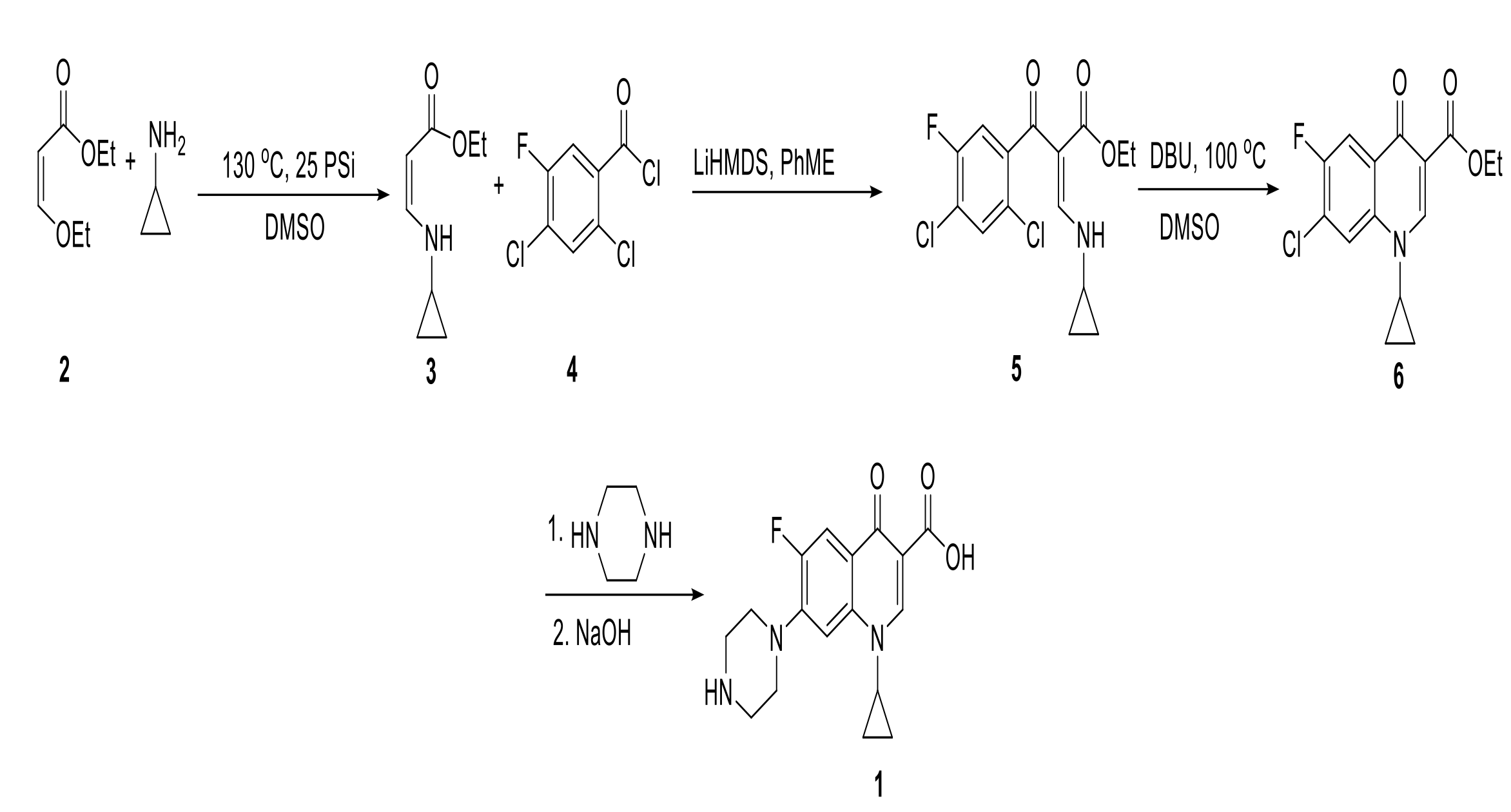
Figure 1. Proposed synthetic route of ciprofloxacin.
