Glaucoma Therapy
Corneal Permeable Anti-Glaucoma Drug
Water soluble, biocompatible pilocarpine analog for treatment of angle-closure glaucoma
Current glaucoma treatments involve topical application of liquid eyedrops, however there are multiple drawbacks to this treatment modality including low bioavailability of target tissue, limited corneal permeability, and poor patient compliance due to frequent application requirements. Therefore, novel antiglaucoma agents that have increased target availability and improved efficacy are required.
The technology
This is a unique compound that could be used to treat a variety of glaucoma conditions. The technology builds on previously commercially available treatment for glaucoma, pilocarpine eye drops. The pilocarpine analog is a pharmaceutically active ionic liquid representing a thermodynamically stable phase. In addition, this ionic liquid form salt could avoid the troublesome issues of polymorphism and “polymorphic transformation”. Liquid eye drop administration remains as the gold standard for drug administration as this technology modified the chemical structure of the pilocarpine to induce ionic properties and improve biocompatibility and corneal permeability.
This technology has been synthesized and characterized in vitro for safety and ex vivo for corneal permeation.
Compared to the commercial pilocarpine hydrochloride (PiloHCl), our new drug (Pilo-OEG) is
- 25-fold safer (non-toxic up to 25 mM compared to 1 mM for commercial PiloHCl),
- 12-fold higher drug permeability through the cornea;
- 2-fold stronger in IOP reduction with longer duration.
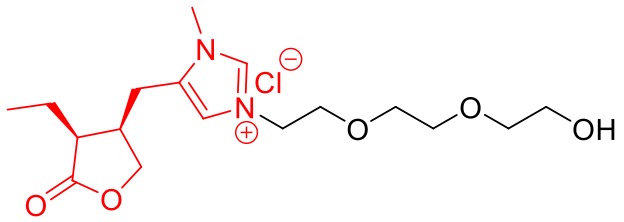
Figure 1. Chemical structure of the novel pilocarpine analog Pilo-OEG.
