RNA Therapeutic
Novel Minimal Circular RNA
Minimal Circular RNA for Vaccine and Therapeutic Use
Messenger RNA (mRNA) therapeutics and vaccines have shown tremendous potential for the treatment or prevention of a variety of diseases such as cancer and more recently SARS-CoV-2. However, the current technology of mRNA therapeutics and vaccines is still limited by the complicated and inefficient enzymatic mRNA production, limited mRNA half-life, and dilution of intracellular mRNA during cell division. Researchers at Virginia Commonwealth University (VCU) have developed a novel method for the production of a unique minimal circular RNA that produces multivalent tandem peptides, thereby remedying these issues.
The technology
This novel method for the production of unique minimal circular RNA (circRNA) and hence the production of multivalent peptides enables the rapid and cost-effective production of RNA therapeutics and vaccines, and efficient and long-lasting peptide production in cells. This method of circRNA production results in a circular RNA that has significantly fewer nucleotides which yields itself to numerous novel aspects including a diminished rate of degradation and a more easily deliverable RNA vaccine. This results in greater vaccine efficacy and preferable safety. Remarkably, these circRNA, which has no stop codon, will produce multivalent tandem peptides via rolling circle translation, which promotes the immunogenicity of RNA vaccines and enhances the binding affinity of the resulting peptide products. In addition, the attributes of the RNA produced results in intrinsic self-adjuvant properties as RNA vaccines that promote the immune responses elicited by these RNA. Because of this, the circRNA vaccines produce a significant humoral and cellular immune response that is often greater than that which is produced by other RNA vaccines. Studies that have evaluated these novel circRNA have shown them to be effective in inducing a more potent T-cell response while also inhibiting tumor progression for the tumor immunotherapy in multiple mouse models, as shown by an example in Figure 1.
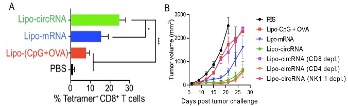
Figure 1. Shown above is (A) the resulting T-cell response after inoculation with liposomes encapsulating either CpG + OVA protein, mRNA expressing whole OVA protein, or the novel circRNA expressing a minimal peptide epitope of OVA; (B) tumor progression over time after treatment with either PBS or liposomes encapsulating either CpG+OVA, mRNA expressing OVA, the novel circRNA expressing a minimal peptide epitope, and the novel circRNA expressing peptide epitope with additional antibody anti-CD4, anti-CD8, or anti-NK1.1 to deplete the T-cell or natural killer cell responses, respectively.
