Cardiovascular Health
Puff Topography Device
Measuring E-cigarette Airflow
Recently the FDA has expanded regulations from the 2009 regulations on cigarettes, smokeless and roll-you-own tobacco to e-cigarettes, cigars and all other tobacco products to ensure consumer protection. Due to public health uncertainty, there is a need to produce a device that measures the effect of delivering nicotine through a vapor as opposed to smoke. Researchers at VCU and American University of Beirut have collaborated to produce a device that can measure puff topography, including but not limited to puff volume, inter-puff interval and puff duration, during e-cigarette use.
The technology
Measurement accuracy is improved over current systems, since this device measures inlet airflow as opposed to outlet airflow at the mouthpiece. Another benefit to measuring inlet airflow is the ability to expand to different designs of e-cigarettes as opposed to specific designs for each mouthpiece. This composite device is portable and easy-to-implement with ranging designs of e-cigarettes, where there are approximately 250 different e-cigarette brands on the market. With the newly approved FDA regulations, there is a need for a puff topography e-cigarette measurement device in order to comply with federal regulations.
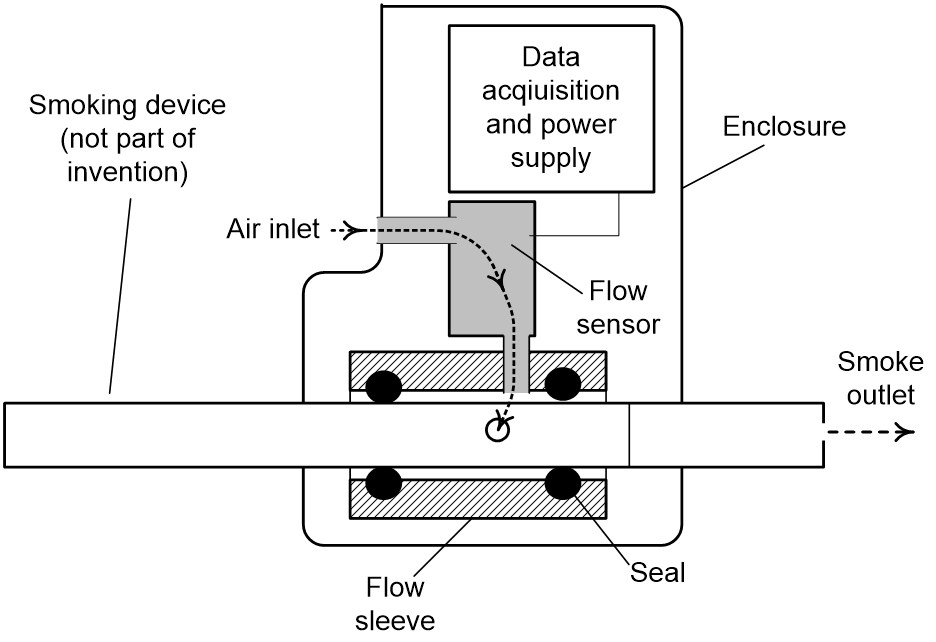
Figure 1. Air flow puff topography apparatus based on measurement of air flow into a smoked device such as an electronic cigarette. Configuration shown for a self-contained portable unit.
