Utilizing Ligated Metal Chalcogenide Clusters as Catalysts to Control Reaction Rates
CO2 Hydrogenation Method
Utilizing Ligated Metal Chalcogenide Clusters as Catalysts to Control Reaction Rates
The greenhouse effect is a phenomenon that causes harmful global environmental changes due to increasing CO2 levels in the earth’s atmosphere. As such, reducing the levels of CO2 through means of capturing the CO2 is a potential solution to mediate the greenhouse effect. Currently, porous or mesoporous absorbents are utilized to capture CO2. However, upscaling this process is often impractical due to the costly CO2 storage and transportation. Thus, researchers at VCU have developed an effective alternative to reducing atmospheric CO2 levels by capturing and converting it into useful bi-products, such as formic acid, via novel ligated metal chalcogenide clusters.
The technology
Converting CO2 to formic acid without an effective catalyst is challenging as CO2 is inherently inert. The use of VCU’s novel metal chalcogenide cluster as a catalyst for CO2 hydrogenation contains a unique capability to alter its energy demand based on the attached ligand (i.e., the barrier height can be controlled by varying the number/type of attached ligands) (Figure 1 and Figure 2). For example, the un-ligated metal chalcogenide cluster produced significantly low barrier heights ranging from 0.3-0.4 electron volts (eV) compared to conventional catalysts. By selectively controlling the ratio of attached acceptor and donor ligands to the metal chalcogenide cluster, one can adjust the barrier heights for CO2 hydrogenation in a stepwise manner. For instance, by attaching only three specific ligands to the cluster, one of the CO2 hydrogenation barriers can be reduced to as low as 0.12 eV that will allow rapid thermochemical conversion of CO2.
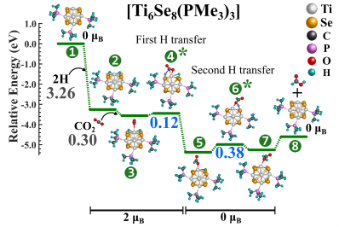
Figure 1. CO2 =>HCOOH reaction pathway on [Ti6Se8(PMe3)3] metal chalcogenide cluster.
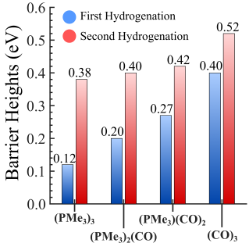
Figure 2. The relative trend of barrier heights for both hydrogenation steps on four ligated clusters.
