Engineering and chemical science
Renewable Hydrogen
Nickel-Molybdenum Alloy Nanoparticles as Low-Cost and Earth-Abundant Electrocatalysts for Water Splitting
Increasing energy demands paired with growing environmental concerns are challenging the current paradigms of power sources and production. Renewable hydrogen is being explored as an energy carrier and chemical reagent to supplement and wean the usage of fossil fuels. Current methods to produce hydrogen include electrochemical water splitting using renewable electricity or direct water splitting via photocatalysis. However, many of the current electro-and photocatalysts for water splitting incorporate costly platinum group metals (PGM) for the hydrogen evolution reaction (HER) as the primary or secondary catalysts. Researchers at VCU have developed an alternative to PGM electrocatalysts using earth-abundant low-cost elements.
The technology
A colloidal synthesis method was developed to produce inexpensive and earth abundant nickel-molybdenum alloy (Ni1-xMox) nanocrystals with sizes ranging from 18–43 nm for alkaline HER (Figure 1). With a water splitting current density of -10 mA/cm2, these alloys demonstrate over-potentials of -62 to -177 mV, which are comparable to commercial Pt-based electrocatalysts (-68 to -129 mV) (Figure 2). The cubic Ni0.934Mo0.066 alloy nanocrystals exhibit the highest activity as alkaline HER electrocatalysts, outperforming commercial Pt/C (20 wt%) catalyst. This platform has the potential to directly replace the current commercially available, expensive, and extremely rare PGM catalysts for renewable and sustainable energy applications.
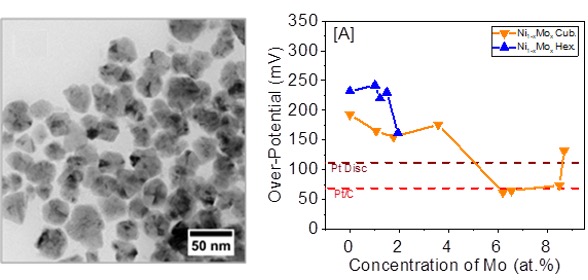
Figure 1. TEM images of 29.9 ± 4.8 nm cubic Ni1-xMox alloy nanocrystals with x = 0.066.
Figure 2. The effect of Mo content on the magnitude of the over-potential for Ni1- xMox alloy nanocrystals at a current density of -10 mA/cm2.
